Organic electrosynthesis is a green synthesis technique that uses electrical energy to drive chemical reactions. In the reaction, electric current is used to replace equivalent chemical oxidation or reduction reagents in traditional synthetic chemistry. At the same time, electrosynthesis also has the advantages of continuous adjustment of current and potential, so it is easy to accurately control the reaction selectivity and reaction rate.
Mei Tian-Sheng research group from State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Science, is dedicated to the cross-research of metal catalysis and organic electrochemistry. In recent years, Mei group has realized the metal-catalyzed C(sp3)–H functionalization via anodic oxidation and the C(sp2)–H functionalization, including amination, alkylation, etc. . In addition, Mei group developed Pd-catalyzed carboxylation of allyl esters and carbon dioxide via cathodic reduction (Fig. 1).
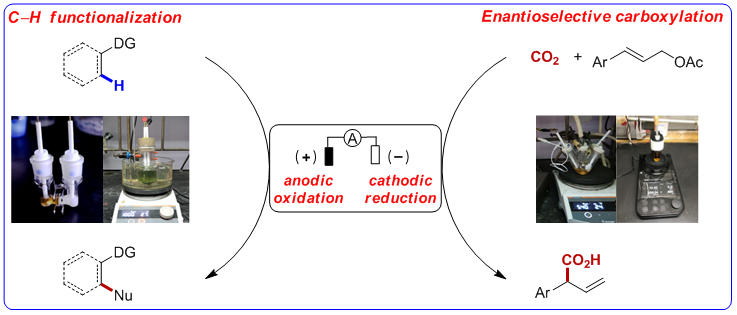
Figure 1. Metal-catalyzed reactions via anodic oxidation or cathodic reduction
Transition-metal-catalyzed coupling reactions are useful tools for synthesizing aryl sulfur compounds. However, conventional transition-metal-catalyzed thiolation of aryl bromides and chlorides typically requires the use of strong base under elevated reaction temperature. Recently, Mei group realized Ni-catalyzed thiolation of aryl and heteroaryl bromides and chlorinated compounds at room temperature by using the "pair-electrolysis" strategy with an undivided cell. The reaction does not require an external base, providing an alternative method for thiolation (Fig 2). These findings provide a new avenue for transition-metal-catalyzed cross-coupling reactions.
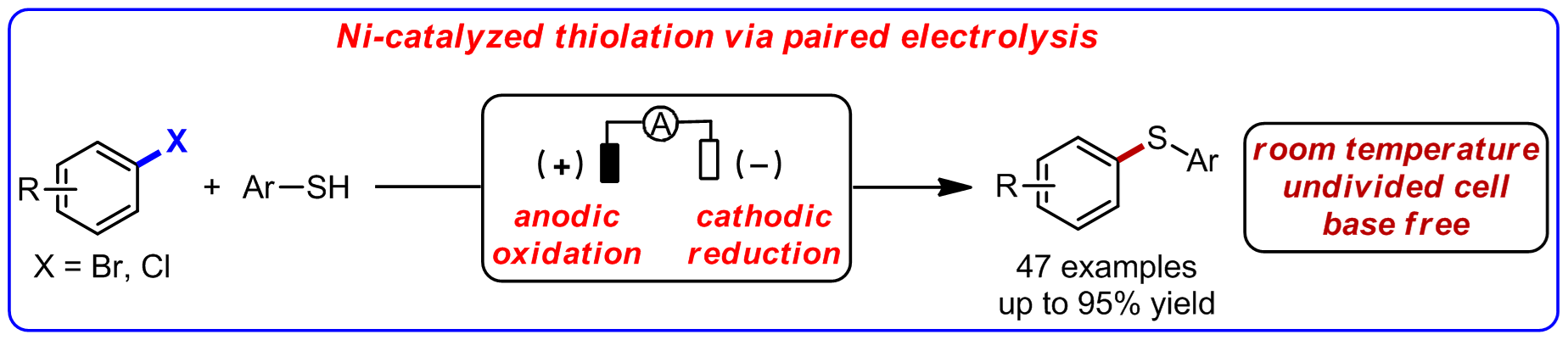
Figure 2. Ni-catalyzed electrochemical thiolation of aryl halides
