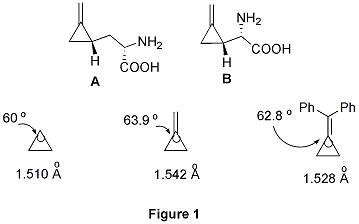
Although they are highly strained, methylenecyclopropanes (MCPs) are readily accessible molecules that have served as useful building blocks in organic synthesis. MCPs can undergo a variety of ring-opening reactions because the release of cyclopropyl ring strain (40 kcal/mol) can provide a thermodynamic driving force for reactions and the p-character of the bonds within the cyclopropane can afford the kinetic opportunity to initiate the ring opening. They can be even included in some natural products such as hypoglycin A and methylenecyclopropylglycine B (Figure 1). Due to the double bond connected to the cyclopropane, the strain of cyclopropane becomes considerably larger than normal cyclopropane, that can be identified by comparing their bond lengths and bond angles (Figure 1).
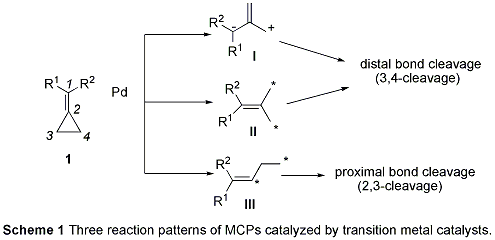
Transition metal-catalyzed reactions of methylenecyclopropanes (MCPs) 1 have been widely explored in this area of study over the past decades. Three kinds of reaction patterns of MCPs 1 have been disclosed for reactions catalyzed by transition metals such as Pd, Rh, Ru, Pt (Scheme 1):
Dr. Min Shi’s group in the State Key Laboratory of Organometallic Chemistry has been systematically investigating the tandem reaction of MCPs. In 2002, they first found the proximal bond cleavage in the presence of Lewis acid (LA) or Brønsted acid (BA), giving novel reaction products (Scheme 2). Moreover, the transition metal can cause the distal bond cleavage, giving a novel cyclobutene derivative when one of R group is a hydrogen atom (Scheme 2). On the other hand, in the presence of LDA or nBuLi (Brønsted base (BB), interesting cascade reaction can take place (Scheme 2). These reports can be recognized in Org. Lett. 2002, 4, 2145. Org. Lett. 2003, 5, 579. Org. Lett. 2003, 5, 1415. Adv. Synth. & Catal. 2003, 345, 963. Chem. Commun. 2004, 2878. Org. Bioorg. Chem. 2005, 3, 399. Chem. Eur. J. 2006, 12, 510. Org. Lett. 2006, 8, 625. J. Am. Chem. Soc. 2006, 128, 7430.Chem. Eur. J. 2007, 13, 862. Org. Lett. 2007, 9, 117. Org. Lett. 2007, 9, 1303. Org. Lett. 2007, 9, 2405. Org. Lett. 2007, 9,4917. Org. Lett. 2007, 9, 5187. Chem. Commun. 2008, 2668. Organometallics 2009, 28, 5600. Dalton Transactions 2010, 39, 8829. Org. Lett. 2010, 12,116. Org. Lett. 2010, 12, 2606. Eur. J. Org. Chem. 2011, 7189.
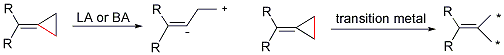
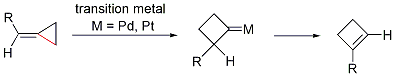
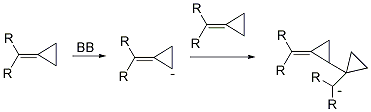
Scheme 2. New reaction patterns of methylidenecyclopropanes
In 2012, they further published rapid generation of molecular complexity in the Lewis or Brønsted acid-mediated reactions of methylenecyclopropanes in Acc. Chem. Res. 2012, 45, 641-652. As shown in Scheme 3, these Lewis or Brønsted acid-mediated reactions of MCPs can produce a variety of new compounds such as cyclobutanones, indenes, tetrahydrofurans, and tetrahydroquinolines. Finally, they have also carried out computational studies to explain the mechanism of the Brønsted acid-mediated reactions of MCPs with acetonitrile.
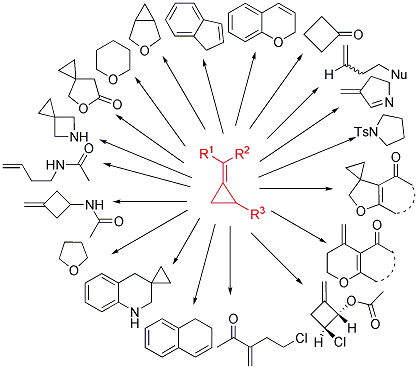
Scheme 3. Rapid generation of molecular complexity from methylenecyclopropanes
Financial support from the Shanghai Municipal Committee of Science and Technology, National Basic Research Program of China (973)-2009CB833302, and the National Natural Science Foundation of China are greatly acknowledged.
