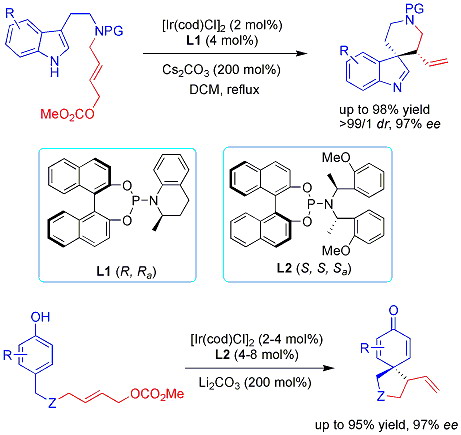
In 2010, the researchers from the State Kay Laboratory of Organometallic Chemistry discovered that an iridium complex could catalyze the asymmetric allylic dearomatization of indoles. With [Ir(cod)Cl]2/phosphoramidite ligand L1, intramolecular C-3 allylic alkylation of indoles has been realized to afford highly enantioenriched spiroindolenine derivatives in excellent yields, drs and ees (Q.-F. Wu, H. He, W.-B. Liu, S.-L. You, J. Am. Chem. Soc. 2010, 132, 11418-11419.). The first iridium-catalyzed asymmetric allylic dearomatization reaction features ready availability of starting materials and a straightforward route to enantioenriched spiroindolenine derivatives.
Phenols are also cheap and abundant chemicals widely used in organic synthesis. Transition-metal-catalyzed allylic alkylation reactions of phenols generally proceeded as O-allylation with limited examples of C-allylation. Recently, the asymmetric allylic dearomatization reaction of phenols was also realized. The enantiopure spirocyclohexadienones obtained here could undergo diverse transformations, and may serve as versatile synthons in organic synthesis (Q.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye, S.-L. You, Angew. Chem. Int. Ed.2011, 50, 4455-4458.).
This project was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology (973 Program), and the Chinese Academy of Sciences.
